Please wait while we confirm your payment. This should take a few seconds...
About the ASCP HT exam
The ASCP Histotechnician (HT) certification exam assesses the knowledge and skills required for entry-level histology positions. The American Society for Clinical Pathology (ASCP) administers the exam.
The exam consists of 100 multiple-choice questions and has a 2.5 hour time limit. The exam covers essential histotechnology topics including tissue fixation, processing, embedding, microtomy, and staining. It also assesses proficiency in laboratory procedures, equipment operation, and safety procedures.
The exam fee is $225.
About these practice questions
These practice questions will help prepare you for the ASCP HT exam.
This page contains 250 practice questions divided into the five sections of the exam: 1. Fixation, 2. Processing, 3. Embedding/Microtomy, 4. Staining, and 5. Laboratory operations.
All questions have been carefully designed to mimic the questions on the real exam, to help you prepare and get a passing grade.
Sections
- Fixation
- Processing
- Embedding/Microtomy
- Staining
- Laboratory operations
Section 1: Fixation
1.1)
How much water must be added to 100 mL of pure bleach to make 10% bleach?
- 700 mL
- 800 mL
- 900 mL
- 1,000 mL
Answers are locked. Please click here to buy them.
1.2)
Which of these reagents contains mercuric chloride?
- Bouin's fluid
- Crystal violet
- Picric acid
- Zenker's fluid
Answers are locked. Please click here to buy them.
1.3)
Why are bacterial smears heat-fixed before staining?
- To make the cell walls permeable so the stain can penetrate
- To melt the bacteria so they release their toxins
- To prevent cells from being washed off during staining
- To provide a warm temperature for the bacteria to grow
Answers are locked. Please click here to buy them.
1.4)
What is the term for formaldehyde dissolved in water?
- Acetone
- Cytospray
- Ethyl acetate
- Formalin
Answers are locked. Please click here to buy them.
1.5)
Smears for cytology must be fixed:
- immediately
- within 2 hours
- within 8 hours
- within 16 hours
Answers are locked. Please click here to buy them.
1.6)
Which of these is a fixative?
- EDTA
- Hydrochloric acid
- Xylene
- Zenker's fluid
Answers are locked. Please click here to buy them.
1.7)
Which of these is a compound fixative?
- Acetic acid
- Bouin's fluid
- Formaldehyde
- Picric acid
Answers are locked. Please click here to buy them.
1.8)
Which of these actions will prevent the formation of acid formaldehyde hematin in formaldehyde solutions?
- Adding drops of cytochrome b5 reductase
- Keeping the solutions refrigerated
- Using buffered formalin
- Washing excess fixative overnight with water
Answers are locked. Please click here to buy them.
1.9)
Which chemical is added to formalin as a stabilizer?
- Half-normal saline
- Iodine
- Methanol
- Sodium phosphate
Answers are locked. Please click here to buy them.
1.10)
What percent of pure formalin is formaldehyde?
- 7–10%
- 17–20%
- 27–30%
- 37–40%
Answers are locked. Please click here to buy them.
1.11)
Which fixative do Pap stains use?
- 10% buffered formalin
- 95% ethanol
- Helly's fixative
- Methanol
Answers are locked. Please click here to buy them.
1.12)
Specimens should be placed in a fixative that is ______ times greater than the volume of the tissue.
- 10–20
- 20–30
- 30–40
- 40–50
Answers are locked. Please click here to buy them.
1.13)
Which chemical is added to formalin as a buffer?
- Half-normal saline
- Iodine
- Methanol
- Sodium phosphate
Answers are locked. Please click here to buy them.
1.14)
How is a 10% solution of stock formalin made?
- 1 part stock formalin with 10 parts water
- 1 part stock formalin with 9 parts water
- 9 parts stock formalin with 1 part water
- 10 parts stock formalin with 1 part water
Answers are locked. Please click here to buy them.
1.15)
During fixation, why is buffered formalin used instead of normal formalin?
- To inactivate cellular enzymes
- To keep the tissue dehydrated
- To maintain a neutral pH
- To prevent cells from bursting
Answers are locked. Please click here to buy them.
1.16)
Fixatives work best at what pH?
- 3
- 5
- 7
- 9
Answers are locked. Please click here to buy them.
1.17)
Which of these fixatives is alcohol-based?
- B-5 fixative
- Carnoy’s fixative
- Formalin
- Helly’s fixative
Answers are locked. Please click here to buy them.
1.18)
Picric acid fixatives such as Bouin's fixative give tissue which color?
- Blue
- Green
- Orange
- Yellow
Answers are locked. Please click here to buy them.
1.19)
In fixation, which molecule is most commonly associated with streaming artifacts?
- Cholesterol
- Glycogen
- Myosin
- Phosphatidylglycerol
Answers are locked. Please click here to buy them.
1.20)
Dark brown granular artifacts are often caused by fixatives containing which chemical?
- Calcium
- Mercury
- Sodium
- Zinc
Answers are locked. Please click here to buy them.
1.21)
What is the preferred fixative for Masson Trichrome?
- Acetone
- Bouin's solution
- Formalin
- Zenker's fixative
Answers are locked. Please click here to buy them.
1.22)
What is autolysis?
- The decay of tissue by bacteria
- The denaturing of proteins by chemical fixation
- The destruction of cells by their own enzymes
- The dissolution of cells by enzymes from another organism
Answers are locked. Please click here to buy them.
1.23)
When transporting tissue specimens in cold weather, how much alcohol should be added to the formalin to prevent the tissue from developing freeze artifacts?
- 1 part alcohol to 3 parts formalin
- 1 part alcohol to 5 parts formalin
- 1 part alcohol to 7 parts formalin
- 1 part alcohol to 9 parts formalin
Answers are locked. Please click here to buy them.
1.24)
In Bouin's solution, what fixative ingredient has a tissue-swelling effect that balances the tissue-shrinking effect of the picric acid?
- Acetic acid
- Chloroform
- Formaldehyde
- Mercuric chloride
Answers are locked. Please click here to buy them.
1.25)
Which of these fixatives is a cross-linking fixative?
- Bouin's solution
- Carnoy's solution
- Ethanol
- Formaldehyde
Answers are locked. Please click here to buy them.
1.26)
What type of fixation artifact occurs when unfixed material in the tissue moves from its original location?
- Chatters
- Floater
- Orientation
- Streaming
Answers are locked. Please click here to buy them.
1.27)
How do cross-linking fixatives preserve tissue?
- By denaturing proteins in the tissue
- By diffusing mitochondria across the cell wall
- By forming bonds between molecules of the tissue
- By overlapping sequence information in strands of DNA
Answers are locked. Please click here to buy them.
1.28)
Buffered commercial formalin is approximately ____ formaldehyde gas by weight/volume.
- 4%
- 10%
- 40%
- 70%
Answers are locked. Please click here to buy them.
1.29)
A tissue slide has holes in the cells and extracellular matrix. What is the cause?
- Formalin pigments
- Ice crystals
- Incomplete decalcification
- Overheating
Answers are locked. Please click here to buy them.
1.30)
What can happen if the heat fixation step is skipped in Gram staining?
- All organisms will appear colorless
- All organisms will appear gram-negative
- All organisms will appear gram-positive
- No cells will appear on the slide
Answers are locked. Please click here to buy them.
Section 2: Processing
2.1)
Anti-CD3 antibodies are used in immunohistochemistry to identify which type of cell?
- B cell
- Mast cell
- Plasmacytoid dendritic cell
- T cell
Answers are locked. Please click here to buy them.
2.2)
In histology, what is xylene used for?
- Attaching coverslips to slides
- Clearing tissue
- Dehydrating tissue
- Fixing autopsy specimens
Answers are locked. Please click here to buy them.
2.3)
Which of these factors would increase the tissue processing time for a histology specimen?
- Dense tissue
- Few pieces of tissue in the tissue cassette
- Porous tissue
- Small specimen size
Answers are locked. Please click here to buy them.
2.4)
What is the correct order of steps to process tissue containing calcium?
- Decalcification, dehydration, fixation, clearing, infiltration
- Decalcification, dehydration, fixation, infiltration, clearing
- Fixation, decalcification, dehydration, clearing, infiltration
- Fixation, dehydration, decalcification, infiltration, clearing
Answers are locked. Please click here to buy them.
2.5)
What does a tissue cassette do?
- Cuts tissue after processing
- Holds tissue during processing
- Keeps tissue refrigerated
- Labels slides
Answers are locked. Please click here to buy them.
2.6)
Which of these is the slowest method of decalcification?
- EDTA
- Formic acid
- Hydrochloric acid
- Nitric acid
Answers are locked. Please click here to buy them.
2.7)
During tissue dehydration, tissue is placed in increasing strengths of which substance?
- Chromic acid
- Ethanol
- Sodium citrate
- Water
Answers are locked. Please click here to buy them.
2.8)
Which ethanol strength is used last when dehydrating tissues?
- 70%
- 80%
- 90%
- 100%
Answers are locked. Please click here to buy them.
2.9)
Which clearing agent does NOT make tissues transparent/translucent?
- Benzene
- Cedarwood oil
- Chloroform
- Xylene
Answers are locked. Please click here to buy them.
2.10)
Which of these is a disadvantage of xylene as a clearing agent?
- Expensive
- Removes aniline dyes
- Slow
- Toxic
Answers are locked. Please click here to buy them.
2.11)
Why do clearing agents need to be miscible with alcohol?
- So they can dehydrate tissues completely
- So they can remove dehydrating agents from tissues
- To prevent damage to the clearing agent
- To prevent damage to the tissue
Answers are locked. Please click here to buy them.
2.12)
What happens if tissue is left in xylene for more than three hours?
- The tissue becomes a milky color
- The tissue becomes brittle
- The tissue becomes red
- The tissue swells in size
Answers are locked. Please click here to buy them.
2.13)
After fixation, bone tissue samples must be:
- decalcified
- frozen
- left at room temperature for at least 24 hours
- refrigerated
Answers are locked. Please click here to buy them.
2.14)
Why are tissues dehydrated before embedding?
- Because wax is hydrophobic
- To make the tissue transparent
- To remove calcium ions from the specimen
- To stop autolysis and putrefaction
Answers are locked. Please click here to buy them.
2.15)
If paraffin is not infiltrated properly, the tissue will:
- become soft and crumbly
- become too hard to cut properly
- liquefy
- lose its color
Answers are locked. Please click here to buy them.
2.16)
What is the purpose of tissue infiltration?
- To give structural support to the tissue
- To keep the cells alive
- To rehydrate the tissue
- To remove bacteria from the tissue
Answers are locked. Please click here to buy them.
2.17)
During tissue processing for histology, what type of water does the dehydration step remove?
- Bound
- Free
- Hard
- Ionized
Answers are locked. Please click here to buy them.
2.18)
Which of these is an advantage of frozen sectioning over paraffin sectioning?
- It is better at preserving antigenicity
- It is resistant to freeze artifacts
- It maintains tissue morphology with less damage
- The sections are more physically stable
Answers are locked. Please click here to buy them.
2.19)
Immunohistochemistry uses antibodies to detect:
- antigens
- enzymes
- lipids
- nucleic acid sequences
Answers are locked. Please click here to buy them.
2.20)
There are white patches on slides after the deparaffinization step. Which of these could be the cause?
- The dye was overoxidized
- The dye was too acidic
- The slides were in xylene for an insufficient amount of time
- The slides were too dry before deparaffinization
Answers are locked. Please click here to buy them.
2.21)
When performing a control for an immunohistochemical test, the positive control should be:
- an antibody of a different isotype to the antibody used in the test
- a tissue that expresses the antigen of interest
- cells from a different species to the cells used in the test
- tissue-specific transcription factors
Answers are locked. Please click here to buy them.
2.22)
Which of these cell block preparation techniques uses heat and is inexpensive?
- Celloidin method
- Gelatin
- Glucomannan-formalin with methanol
- Picric acid
Answers are locked. Please click here to buy them.
2.23)
Overdehydration of tissue can cause what type of artifact?
- Fulguration
- Shrinkage
- Squeeze
- Streaming
Answers are locked. Please click here to buy them.
2.24)
At what speed and for how long is the sample centrifuged in the plasma thrombin method of preparing cell blocks?
- 1–2 minutes at 2500–3000 rpm
- 1–2 minutes at 5500–6000 rpm
- 5–10 minutes at 2500–3000 rpm
- 5–10 minutes at 5500–6000 rpm
Answers are locked. Please click here to buy them.
2.25)
In immunohistochemistry, blocking prevents:
- antibody-antigen interactions
- cross-linking of proteins
- loss of antigenicity
- nonspecific antibody binding
Answers are locked. Please click here to buy them.
2.26)
What is the expected result of a negative control of an immunohistochemical test?
- No specific staining
- Staining of all cells
- Staining of cells expected to express the antigen
- Staining of cells without the antigen
Answers are locked. Please click here to buy them.
Section 3: Embedding/Microtomy
3.1)
What is the most widely used medium for infiltration and embedding?
- Formic acid
- Paraffin wax
- Permount
- Xylene
Answers are locked. Please click here to buy them.
3.2)
Paraffin wax should be used at ___________ its melting point.
- 5–10°C below
- 2–3°C below
- the same temperature as
- 2–3°C above
Answers are locked. Please click here to buy them.
3.3)
An advantage of paraffin wax embedding is:
- it preserves fats
- samples can be stored long-term
- sections can be cut ultra-thin
- tissue can be viewed without a microscope
Answers are locked. Please click here to buy them.
3.4)
What is the melting point of Paraplast?
- 52–56°C
- 62–66°C
- 72–76°C
- 82–86°C
Answers are locked. Please click here to buy them.
3.5)
How long can formalin-fixed paraffin-embedded (FFPE) tissue be stored?
- 1-2 weeks
- 1-2 months
- 6-12 months
- Indefinitely
Answers are locked. Please click here to buy them.
3.6)
Why are tissues embedded in paraffin wax?
- So they can be cut into sections
- So they can be fixed in formalin
- To decalcify them
- To kill microorganisms
Answers are locked. Please click here to buy them.
3.7)
Sectioning is the process of:
- cutting tissue into thin slices
- infiltrating tissue samples with paraffin
- preserving biological tissues from decay
- removing dehydrating agents from tissues
Answers are locked. Please click here to buy them.
3.8)
Tissue flotation baths are set at what temperature?
- 30–40°C
- 40–50°C
- 50–60°C
- 60–70°C
Answers are locked. Please click here to buy them.
3.9)
Tissues are sectioned using a:
- coverslipper
- microtome
- tissue embedding center
- tissue processor
Answers are locked. Please click here to buy them.
3.10)
Microtome blades should generally be angled at around:
- 5 degrees
- 15 degrees
- 25 degrees
- 35 degrees
Answers are locked. Please click here to buy them.
3.11)
Which of these is a disadvantage of paraffin wax embedding?
- Difficult storage
- Poor subsequent staining with H&E
- The inability to cut very thin sections
- The inability to ribbon
Answers are locked. Please click here to buy them.
3.12)
Microtomes can cut tissue at thicknesses varying from 2 to 50 ________
- nanometers
- micrometers
- millimeters
- centimeters
Answers are locked. Please click here to buy them.
3.13)
A freezing microtome is also known as a:
- cryostat
- hand microtome
- sledge microtome
- ultramicrotome
Answers are locked. Please click here to buy them.
3.14)
Infiltration is the:
- permeation of tissue with ethanol
- permeation of tissue with wax
- removal of calcium from tissue
- removal of water from tissue
Answers are locked. Please click here to buy them.
3.15)
Which microtome is used to prepare tissue sections for electron microscopy?
- Cryostat
- Rotary microtome
- Sledge microtome
- Ultramicrotome
Answers are locked. Please click here to buy them.
3.16)
Which freezing technique is the coldest?
- Aerosol sprays
- Carbon dioxide gas
- Carbon dioxide ‘cardice'
- Liquefied nitrogen
Answers are locked. Please click here to buy them.
3.17)
A lab technician has cut a ribbon of sections from a tissue block. What should the lab technician do next?
- Add a few drops of a softening agent
- Float the sections on warm water
- Leave the sections in a freezer for an hour
- Submerge the sections in ethanol
Answers are locked. Please click here to buy them.
3.18)
What is the most common type of microtome in laboratories?
- Cryostat
- Rotary microtome
- Sledge microtome
- Ultramicrotome
Answers are locked. Please click here to buy them.
3.19)
Diamond blades are needed to cut:
- bone
- intestinal tissue
- muscle tissue
- skin tissue
Answers are locked. Please click here to buy them.
3.20)
Which blade can NOT cut tissue for electron microscopy?
- Gem-quality diamond
- Glass
- Industrial-grade diamond
- Steel
Answers are locked. Please click here to buy them.
3.21)
Plastic sections used in transmission electron microscopy are typically cut how thick?
- 60–100 nm
- 60–100 μm
- 6–10 μm
- 6–10 mm
Answers are locked. Please click here to buy them.
3.22)
Wax sections are typically cut how thick during microtomy?
- 4–10 nm
- 40–100 nm
- 4–10 µm
- 4–10 mm
Answers are locked. Please click here to buy them.
3.23)
For electron microscopy, sections must be about _______ times thinner than sections for light microscopy.
- 2
- 20
- 200
- 2,000
Answers are locked. Please click here to buy them.
3.24)
A histologist is cutting tissue into sections using a microtome. However, the tissue sections keep sticking to the block on the return stroke. Which of the following actions would correct the problem?
- Cool the block with ice
- Decalcify the block
- Increase the clearance angle
- Tighten the knife in the holder
Answers are locked. Please click here to buy them.
3.25)
A lab technician cuts paraffin sections and places them in a water bath. However, the sections do not flatten properly and remain wrinkled. What should the lab technician do?
- Change the water in the water bath
- Check the water bath for air bubbles
- Increase the temperature of the water bath
- Place the sections in a vacuum integration bath
Answers are locked. Please click here to buy them.
3.26)
"Chatters" refer to:
- fragments of bone dust in the specimen
- holes in epithelium and tissue spaces
- intracellular clefts and vacuoles in skeletal muscle
- thick or thin zones in the tissue parallel to the knife edge
Answers are locked. Please click here to buy them.
3.27)
Which embedding medium is used for electron microscopy studies?
- Agarose
- Gelatin
- Paraffin
- Resin
Answers are locked. Please click here to buy them.
3.28)
Which type of microtome is used to cut celloidin?
- Laser
- Saw
- Sliding
- Vibrating
Answers are locked. Please click here to buy them.
3.29)
What does the H&E staining method use as a dewaxing agent?
- Eosin
- Formalin
- Hematoxylin
- Xylene
Answers are locked. Please click here to buy them.
Section 4: Staining
4.1)
Which Gram stain reagent acts as a mordant to bind the stain to the bacteria?
- Acetone
- Ethanol
- Iodine
- Safranin
Answers are locked. Please click here to buy them.
4.2)
__________ staining is the process of staining living cells that have been removed from the body.
- Gram
- In vivo
- Positive
- Supravital
Answers are locked. Please click here to buy them.
4.3)
Which of these stains is supravital?
- New methylene blue
- Romanowsky stain
- Wright's stain
- Ziehl-Neelsen stain
Answers are locked. Please click here to buy them.
4.4)
Which of these could cause stain precipitation?
- Excessive buffer
- Excessive humidity in the air
- Insufficient staining time
- Use of aged staining solutions
Answers are locked. Please click here to buy them.
4.5)
What are the two dyes in the Romanowsky stain?
- Carbol fuchsin and methylene blue
- Crystal violet and carbol fuchsin
- Crystal violet and safranin
- Methylene blue and eosin
Answers are locked. Please click here to buy them.
4.6)
Giemsa's solution is a mixture of:
- eosin, methylene blue, and azure B
- eosin, safranin, and hematoxylin
- phloxine, methylene blue, and hematoxylin
- safranin, azure B, and hematoxylin
Answers are locked. Please click here to buy them.
4.7)
What is the decolorizing agent in the Ziehl Neelsen stain?
- Absolute alcohol
- Acetone
- Acid alcohol
- Xylol
Answers are locked. Please click here to buy them.
4.8)
Identify the false statement about the preparation of reticulocyte smears.
- Reticulocyte smears are used to determine the number of white blood cells in a sample
- The dyes used can be new methylene blue or brilliant cresyl blue
- The stain and the cells must be left to react for 15 minutes before making the smear
- The staining technique is called supravital staining
Answers are locked. Please click here to buy them.
4.9)
In regressive staining, tissue sections are ___________ and then differentiated with dilute acid until the optimal endpoint is reached.
- dehydrated
- hydrated
- overstained
- sectioned
Answers are locked. Please click here to buy them.
4.10)
Before staining, paraffin sections must be:
- dewaxed
- left underwater for at least an hour
- refrigerated
- submerged in a chlorine solution
Answers are locked. Please click here to buy them.
4.11)
In the hematoxylin and eosin (H&E) staining method, the eosin stains:
- connective tissue blue
- cytoplasms pink
- microorganisms blue
- nuclei pink
Answers are locked. Please click here to buy them.
4.12)
When a smear is too red, neutrophil granules look:
- blue-red
- brilliant red
- indistinct
- light blue
Answers are locked. Please click here to buy them.
4.13)
New methylene blue reagent is used to stain:
- Heinz bodies
- eosinophils
- platelets
- reticulocytes
Answers are locked. Please click here to buy them.
4.14)
Which type of bacteria does the Ziehl-Neelsen stain identify?
- Acid-fast
- Facultative anaerobic
- Gram-negative
- Spore-forming
Answers are locked. Please click here to buy them.
4.15)
What is the counterstain in Gram's technique?
- Acetone-alcohol
- Crystal violet
- Iodine
- Safranin
Answers are locked. Please click here to buy them.
4.16)
What is the correct order of reagents in Gram staining?
- Crystal violet, iodine, acetone-alcohol, safranin
- Crystal violet, safranin, acetone-alcohol, iodine
- Iodine, crystal violet, acetone-alcohol, safranin
- Safranin, iodine, crystal violet, acetone-alcohol
Answers are locked. Please click here to buy them.
4.17)
Why are bacterial smears heat-fixed before staining?
- To make the cell walls visible under the microscope
- To prevent the cells from washing off the slide during staining
- To provide a warm temperature for the bacteria to grow
- To remove alcohol from the slide
Answers are locked. Please click here to buy them.
4.18)
Which of these stains is a Romanowsky stain?
- Gram
- Jefferson
- Leishman
- Sudan
Answers are locked. Please click here to buy them.
4.19)
What cell structure turns blue in H&E staining?
- Cytoplasm
- Golgi apparatus
- Nuclei
- Reticulum
Answers are locked. Please click here to buy them.
4.20)
The most common mordant for H&E staining is:
- Alcian blue
- alum
- eosin
- sodium metabisulfite
Answers are locked. Please click here to buy them.
4.21)
What is the decolorizer in H&E staining?
- Acid-alcohol
- Eosin
- Orange G
- Peroxide
Answers are locked. Please click here to buy them.
4.22)
Which two stains are routinely used in Pap stains?
- Hematoxylin and eosin
- Methenamine silver and mucicarmine
- Periodic acid-Schiff-diastase and azur
- Perl's Prussian blue and mucicarmine
Answers are locked. Please click here to buy them.
4.23)
The Pap stain stains which structure blue, purple, or black?
- Cytoplasm
- Golgi apparatus
- Mitochondria
- Nuclei
Answers are locked. Please click here to buy them.
4.24)
What is the ratio of eosin to methylene blue in eosin methylene blue?
- 1:1
- 1:2
- 3:1
- 6:1
Answers are locked. Please click here to buy them.
4.25)
Which of these dyes is alkaline?
- Acid fuchsin
- Carbol fuchsin
- Eosin
- Orange G
Answers are locked. Please click here to buy them.
4.26)
Which of these dyes is acidic?
- Basic fuchsin
- Crystal violet
- Eosin
- Safranin
Answers are locked. Please click here to buy them.
4.27)
Mucicarmine is used primarily to stain:
- elastic fibers
- epithelial mucin
- fat
- nervous tissue
Answers are locked. Please click here to buy them.
4.28)
Which stain can differentiate collagen fibers from other fibers?
- Hematoxylin and eosin stain
- Masson's trichrome stain
- Reticular stain
- Sudan stain
Answers are locked. Please click here to buy them.
4.29)
Which of these stains is a trichrome stain?
- Gomori
- Papanicolaou
- Romanowsky
- Verhoeff-van Gieson
Answers are locked. Please click here to buy them.
4.30)
Which solution in Masson's trichrome stain is sometimes referred to as the fiber stain?
- Ferric chloride in hydrochloric acid
- Hematoxylin in 95% ethanol
- Solution B
- Solution C
Answers are locked. Please click here to buy them.
4.31)
Lillie's trichrome uses which dye for the plasma stain?
- Biebrich scarlet
- Congo red
- Eosin
- Xylidine ponceau
Answers are locked. Please click here to buy them.
4.32)
Which element does Perls Prussian blue detect?
- Iron
- Lead
- Mercury
- Potassium
Answers are locked. Please click here to buy them.
4.33)
Which stain detects amyloids?
- Biebrich scarlet
- Congo red
- Eosin
- Xylidine ponceau
Answers are locked. Please click here to buy them.
4.34)
The Grocott-Gomori stain is used widely as a screen for:
- HIV
- fungi
- influenza
- salmonella
Answers are locked. Please click here to buy them.
4.35)
Van Gieson's stain is a mixture of picric acid and:
- acid fuchsin
- methylene blue
- thionine
- toluidine blue
Answers are locked. Please click here to buy them.
4.36)
The HvG stain is a combination of Van Gieson's stain and which dye?
- Crystal violet
- Giemsa
- Hematoxylin
- Safranin
Answers are locked. Please click here to buy them.
4.37)
At which of these pH levels is Alcian blue solution used?
- 2.5
- 4.5
- 6.5
- 8.5
Answers are locked. Please click here to buy them.
4.38)
What color is acid fuchsin?
- Black
- Green
- Orange
- Red
Answers are locked. Please click here to buy them.
4.39)
What color are red blood cells after Giemsa staining?
- Dark blue
- Pale blue
- Pale pink
- Violet
Answers are locked. Please click here to buy them.
4.40)
Masson's trichrome staining stains muscle fibers which color?
- Black
- Green
- Pink
- Red
Answers are locked. Please click here to buy them.
4.41)
Which stain is used to stain lipids?
- Gram's stain
- H&E
- Periodic Acid-Schiff
- Sudan IV
Answers are locked. Please click here to buy them.
4.42)
Which stain is used to stain glycogen?
- Gram's stain
- H&E
- Periodic Acid-Schiff
- Sudan IV
Answers are locked. Please click here to buy them.
4.43)
Alcian blue stains what part of goblet cells?
- DNA
- Golgi apparatus
- Mucins
- RNA
Answers are locked. Please click here to buy them.
4.44)
The Pap stain stains keratin which color?
- Black
- Blue
- Green
- Orange
Answers are locked. Please click here to buy them.
4.45)
In acid-fast staining, what color are acid-fast bacteria?
- Blue
- Green
- Red
- Yellow
Answers are locked. Please click here to buy them.
4.46)
The Pap stain helps detect which disease?
- Cervical cancer
- Cushing's syndrome
- Muscular dystrophy
- Pernicious anemia
Answers are locked. Please click here to buy them.
4.47)
Which of these is a common ripening agent for hematoxylin?
- Hydrochloric acid
- Iron
- Sodium iodate
- Tungsten
Answers are locked. Please click here to buy them.
4.48)
Hematoxylin ripens into:
- hematein
- hematone
- hematurg
- hemoglobin
Answers are locked. Please click here to buy them.
4.49)
A lab technician stains a blood film using Wright's stain. However, under the microscope, the red blood cells appear excessively red and the nuclei are poorly stained. What could be the cause?
- Inadequate washing
- Prolonged staining
- The pH of the buffer was too high
- The stain/buffer mixture was too acidic
Answers are locked. Please click here to buy them.
4.50)
A section was prepared with H&E staining. Under the microscope, the nuclei are red instead of blue. Which of these could be the cause?
- The eosin solution is too concentrated
- The hematoxylin is breaking down
- The section was not stained long enough in hematoxylin
- The section was stained too long in eosin
Answers are locked. Please click here to buy them.
4.51)
A histologist is performing paraffin H&E staining on a blood smear. When the histologist observes the specimen under a microscope, the basophils are stained too lightly and are difficult to see. What is the most likely cause?
- Incomplete deparaffinization
- The acid alcohol concentration was too low
- The hematoxylin was too strong
- The specimen was left in the stain for too long
Answers are locked. Please click here to buy them.
4.52)
A section is prepared with H&E staining. Under the microscope, the nuclei appear too pale. Which of these could be the cause?
- Insufficient dehydration
- The pH of the eosin solution was above 5.0
- The section was not dried properly before beginning deparaffinization
- Understaining with hematoxylin
Answers are locked. Please click here to buy them.
4.53)
A slide stained with H&E stain appears cloudy. What is the cause?
- Excessive exposure of the specimen to alcohol
- Insufficient clearing
- The stain was cold
- The stain was too hot
Answers are locked. Please click here to buy them.
4.54)
A histologist has stained a blood sample with Wright's stain but the basophils have poor definition. The histologist decides to repeat the stain. Which of these actions would improve the definition?
- Decrease the concentration of the stain/buffer solution
- Dilute the stain with methanol or water
- Reduce the time between preparing the smear and fixation
- Wash the slide before fixation
Answers are locked. Please click here to buy them.
4.55)
Sections are prepared with H&E staining. However, there is a bluish-black precipitate on top of the sections. Which of these could be the cause?
- The hematoxylin solution was not filtered
- The sections were not completely dehydrated
- The sections were stained too long in eosin
- The sections were stained too long in hematoxylin
Answers are locked. Please click here to buy them.
4.56)
What is the sequence of steps for Gram staining?
- Primary stain, decolorizing, counterstain, mordant
- Primary stain, decolorizing, mordant, counterstain
- Primary stain, mordant, counterstain, decolorizing
- Primary stain, mordant, decolorizing, counterstain
Answers are locked. Please click here to buy them.
4.57)
After Gram staining, what color are gram-negative organisms?
- Dark blue
- Light blue
- Purple or brown
- Red or pink
Answers are locked. Please click here to buy them.
4.58)
What color are gram-positive organisms after a Gram stain?
- Orange
- Purple
- Red
- Yellow
Answers are locked. Please click here to buy them.
4.59)
The cell walls of gram-positive bacteria have what property that enables them to resist decolorization during Gram staining?
- A high mycolic acid content
- A lipid bilayer
- A thick layer of peptidoglycan
- Multiple endoflagella
Answers are locked. Please click here to buy them.
4.60)
In Gram staining, which of these situations can cause gram-negative cells to appear colorless?
- Omission of the counterstain step
- Omission of the iodine treatment step
- Over-decolorizing
- Under-decolorizing
Answers are locked. Please click here to buy them.
4.61)
In Gram staining, what can happen if the decolorizer is left on too long?
- All organisms will appear colorless
- Gram-negative organisms will look gram-positive
- Gram-positive organisms will look gram-negative
- No cells will appear on the slide
Answers are locked. Please click here to buy them.
4.62)
A lab technician performs Gram staining of a urine specimen. Under a microscope, the lab technician sees gram-negative cocci in chains. What mistake did the lab technician make?
- Forgot the iodine treatment step
- Forgot to bring the urine specimen to room temperature
- Performed the safranin counterstain too quickly
- Used too much crystal violet
Answers are locked. Please click here to buy them.
4.63)
What are the three common types of acid-fast staining?
- Calcofluor, fast green, and Gram-Twort
- Periodic acid-Schiff, alcian, and Bismarck
- Trichrome, sulfate, and Wade-Fite
- Ziehl-Neelsen, Kinyoun, and auramine-rhodamine
Answers are locked. Please click here to buy them.
4.64)
Which acid-fast staining method is known as the hot method?
- Auramine-Rhodamine
- Fluorochrome
- Kinyoun
- Ziehl-Neelsen
Answers are locked. Please click here to buy them.
4.65)
Which stain differentiates types of blood cells?
- Gram
- H&E
- Papanicolaou
- Romanowsky
Answers are locked. Please click here to buy them.
Section 5: Laboratory operations
5.1)
A molar solution is:
- 1 mL of solute in one mole of solution
- one gram of solute in 1 mL of solution
- one gram of solute in one mole of solution
- one mole of solute in 1 liter of solution
Answers are locked. Please click here to buy them.
5.2)
What is the dilution factor 1/5 expressed as a dilution ratio?
- 1:4
- 1:5
- 1:6
- 5:1
Answers are locked. Please click here to buy them.
5.3)
A lab technician is making a solution by combining 3 parts of a solute with 7 parts of water. How many milliliters of the solute and water are needed to make 100 mL of the solution?
- 7 mL of solute and 3 mL of water
- 10 mL of solute and 70 mL of water
- 30 mL of solute and 70 mL of water
- 70 mL of solute and 30 mL of water
Answers are locked. Please click here to buy them.
5.4)
If a dilution ratio of 7:10 is used to make an acetic acid solution and the final volume of the solution is 950 mL, what is the volume of the solute?
- 58 mL
- 101 mL
- 391 mL
- 559 mL
Answers are locked. Please click here to buy them.
5.5)
A lab technician is preparing a serial dilution with eight tubes. The first tube contains a 1/2 dilution. If the dilution factor doubles with each transfer, what would be the dilution in the eighth tube?
- 1/128
- 1/256
- 1/512
- 1/1024
Answers are locked. Please click here to buy them.
5.6)
A lab technician makes a solution by mixing 500 mL of water with 4.5 g of salt. What is the salt concentration of the solution?
- 0.9 g/L
- 8 g/L
- 8.9 g/L
- 9 g/L
Answers are locked. Please click here to buy them.
5.7)
If 100% bleach is diluted 1/5 and then diluted again 1/20, what is the concentration of bleach in the final solution?
- 1%
- 1.5%
- 2%
- 2.5%
Answers are locked. Please click here to buy them.
5.8)
Calculate the volume of a 0.5 mol/dm³ solution that contains 1 mol of solute.
- 0.5 dm³
- 0.75 dm³
- 1 dm³
- 2 dm³
Answers are locked. Please click here to buy them.
5.9)
A NaOH solution has a relative formula mass of 40 and a 4 g/dm³ concentration. What is the concentration in mol/dm³?
- 0.1
- 2
- 20
- 80
Answers are locked. Please click here to buy them.
5.10)
If 20 moles of A are dissolved in 80 moles of B, what is the mole fraction of A in the final solution?
- 0.2
- 4
- 15
- 60
Answers are locked. Please click here to buy them.
5.11)
What is the symbol for molality?
- M
- m
- mM
- n
Answers are locked. Please click here to buy them.
5.12)
If a solution has a concentration of 10 g/dm³ and the molecules in the solution have a molar mass of 32, what is the concentration of the solution in mol/dm³?
- 0.3125
- 3.2
- 22
- 320
Answers are locked. Please click here to buy them.
5.13)
What does the unit mol/kg represent?
- Milli-molarity
- Molality
- Molar mass
- Molarity
Answers are locked. Please click here to buy them.
5.14)
0.5 mol of solute is dissolved in 250 cm³ of solution. What is the concentration in mol/dm³?
- 0.002 mol/dm³
- 2 mol/dm³
- 12.5 mol/dm³
- 125 mol/dm³
Answers are locked. Please click here to buy them.
5.15)
5 moles of CaCl₂ are dissolved in 1 liter of water. What is the molarity of this solution?
- 0.2
- 1
- 4.8
- 5
Answers are locked. Please click here to buy them.
5.16)
What is the mass percent of a solution containing 25 g of glucose in 500 g of solution?
- 5%
- 10%
- 15%
- 20%
Answers are locked. Please click here to buy them.
5.17)
How many milliliters of 5% hydrochloric acid are needed to make 500 mL of 2% hydrochloric acid?
- 100
- 200
- 300
- 400
Answers are locked. Please click here to buy them.
5.18)
A lab technician needs 300 mL of 5% acetic acid but only has pure acetic acid. How much pure acetic acid and how much water does the technician need to make the required solution?
- 5 mL of pure acetic acid and 295 mL of water
- 10 mL of pure acetic acid and 290 mL of water
- 15 mL of pure acetic acid and 285 mL of water
- 20 mL of pure acetic acid and 280 mL of water
Answers are locked. Please click here to buy them.
5.19)
A lab technician requires 400 mL of a 3% solution but has only a 5% solution available. How would the lab technician prepare the 3% solution?
- Mix 240 mL of the 5% solution with 160 mL of diluent
- Mix 300 mL of the 5% solution with 100 mL of diluent
- Mix 360 mL of the 5% solution with 40 mL of diluent
- Mix 380 mL of the 5% solution with 20 mL of diluent
Answers are locked. Please click here to buy them.
5.20)
How many grams of sodium chloride are needed to make 300 mL of a 2% m/v solution?
- 2
- 4
- 6
- 20
Answers are locked. Please click here to buy them.
5.21)
How would you prepare 400 mL of a 1% v/v acetic acid solution?
- 1 mL of glacial acetic acid in 399 mL of water
- 2 mL of glacial acetic acid in 398 mL of water
- 3 mL of glacial acetic acid in 397 mL of water
- 4 mL of glacial acetic acid in 396 mL of water
Answers are locked. Please click here to buy them.
5.22)
What does 5% w/v mean?
- 5 grams of solute dissolved in 100 mL of solution
- 5 grams of solute dissolved in 100 mL of solvent
- 5 mL of solute dissolved in 100 mL of solution
- 5 mL of solute dissolved in 100 mL of solvent
Answers are locked. Please click here to buy them.
5.23)
Which of these expresses solution concentration for a solid solute dissolved in a liquid solvent?
- %(m/m)
- %(m/v)
- %(v/m)
- %(v/v)
Answers are locked. Please click here to buy them.
5.24)
What is the formula for calculating a percent w/v solution?
- (solute mass / solution mass) × 100
- (solute mass / solution volume) × 100
- (solution volume / solute mass) × 100
- (solution volume / solute volume) × 100
Answers are locked. Please click here to buy them.
5.25)
Which of these is NOT an acceptable way to express solution concentration?
- volume/volume (v/v)
- volume/weight (v/w)
- weight/volume (w/v)
- weight/weight (w/w)
Answers are locked. Please click here to buy them.
5.26)
Calculate the %(m/m) of a solution that has 6 g of solute in 80 g of solution.
- 7.5%
- 8.1%
- 12.3%
- 13.3%
Answers are locked. Please click here to buy them.
5.27)
A mg is a unit to describe:
- length
- mass
- time
- volume
Answers are locked. Please click here to buy them.
5.28)
Which of these units is the smallest?
- Meter
- Micrometer
- Millimeter
- Nanometer
Answers are locked. Please click here to buy them.
5.29)
What is the SI unit for volume?
- Cubic decimeter
- Cubic meter
- Liter
- Milliliter
Answers are locked. Please click here to buy them.
5.30)
Which of these units is the largest?
- Gram
- Kilogram
- Microgram
- Milligram
Answers are locked. Please click here to buy them.
5.31)
What is the basic metric unit for measuring weight?
- Gram
- Liter
- Pascal
- Pound
Answers are locked. Please click here to buy them.
5.32)
What is the basic metric unit for measuring length?
- Foot
- Inch
- Meter
- Meter cubed
Answers are locked. Please click here to buy them.
5.33)
What does the prefix kilo- mean?
- 10
- 100
- 1,000
- 10,000
Answers are locked. Please click here to buy them.
5.34)
Which prefix means 0.001?
- Micro-
- Centi-
- Pico-
- Milli-
Answers are locked. Please click here to buy them.
5.35)
What does the prefix nano- mean?
- 1 hundredth
- 1 thousandth
- 1 millionth
- 1 billionth
Answers are locked. Please click here to buy them.
5.36)
200 microliters is how many milliliters?
- 0.2 mL
- 2 mL
- 20 mL
- 2,000 mL
Answers are locked. Please click here to buy them.
5.37)
If a red blood cell is 8 micrometers in diameter, what is its size in millimeters?
- 0.008 mm
- 0.8 mm
- 80 mm
- 8,000 mm
Answers are locked. Please click here to buy them.
5.38)
A millimeter is a:
- tenth of a meter
- hundredth of a meter
- thousandth of a meter
- millionth of a meter
Answers are locked. Please click here to buy them.
5.39)
The TLV-C is the concentration of a substance that:
- a worker can be exposed to for one hour without adverse effect
- a worker can be exposed to over a working lifetime without adverse effects
- a worker can be exposed to up to 4 times per day
- should never be exceeded
Answers are locked. Please click here to buy them.
5.40)
What is the name of a document that identifies hazards and the ways to minimize their impact?
- Accident and incident recording form
- First aid assessment form
- Inspection report
- Risk management policy
Answers are locked. Please click here to buy them.
5.41)
Which of these actions helps prevent repetitive strain injuries at a computer?
- Keep your wrists bent while typing
- Position the monitor at a low height
- Rest your forearms on the desk for support while typing
- Sit with your feet flat on the floor
Answers are locked. Please click here to buy them.
5.42)
Which of these actions helps prevent the release of aerosols in the laboratory?
- Filling centrifuge tubes to the brim
- Forcibly expelling hazardous materials from pipettes
- Opening aerosol containment devices immediately after removing them from the centrifuge
- Routinely inspecting the centrifuge to ensure there is no leakage
Answers are locked. Please click here to buy them.
5.43)
When trying to fix an electrical machine, you should first:
- replace old cables
- replace the fuses
- turn off all electricity to the lab
- turn the machine off
Answers are locked. Please click here to buy them.
5.44)
How should you pick up an item of hot glassware?
- By pouring cold water on it to cool it down
- With a rag or paper towels
- With tongs
- With your hands
Answers are locked. Please click here to buy them.
5.45)
Which of these liquids is flammable?
- Bleach
- Ethanol
- Nitric acid
- Saline
Answers are locked. Please click here to buy them.
5.46)
Which type of fire are CO₂ fire extinguishers effective against?
- Flammable gas
- Flammable liquid
- Paper
- Wood
Answers are locked. Please click here to buy them.
5.47)
Which of these is the most flammable?
- Acids
- Organic solvents
- Strong oxidizers
- Strong reducers
Answers are locked. Please click here to buy them.
5.48)
Which rating of fire extinguisher is used in chemical fires?
- A
- B
- C
- D
Answers are locked. Please click here to buy them.
5.49)
Which of these is an example of passive fire protection?
- Fire alarms
- Fire extinguishers
- Fire-resistant walls
- Sprinklers
Answers are locked. Please click here to buy them.
5.50)
Which of these chemicals is flammable?
- Acetone
- Carbon tetrachloride
- Chloroform
- Saline
Answers are locked. Please click here to buy them.
5.51)
At low concentrations, what does hydrogen sulfide smell like?
- Burnt sugar
- Rotten eggs
- Rotting meat
- Strong cheese
Answers are locked. Please click here to buy them.
5.52)
Which of these chemicals is corrosive to skin?
- Calcium acetate
- Hydrogen peroxide
- Potassium phosphate
- Sodium chloride
Answers are locked. Please click here to buy them.
5.53)
Why is mercury hazardous?
- It forms an explosive precipitate on standing
- It forms toxic vapors
- It is flammable
- It reacts violently with water
Answers are locked. Please click here to buy them.
5.54)
What is the term for chemicals that cause birth defects in embryos and fetuses?
- Carcinogens
- Corrosives
- Phenyls
- Teratogens
Answers are locked. Please click here to buy them.
5.55)
When handling strong acids in a laboratory:
- add water to acid when diluting
- wear eye protection
- wear latex gloves
- wear open-toed shoes
Answers are locked. Please click here to buy them.
5.56)
Chemicals that produce harmful vapors should be used in a:
- autoclave
- biological safety cabinet
- fume hood
- sealed room
Answers are locked. Please click here to buy them.
5.57)
What is a term for chemicals that burn or destroy tissue?
- Carcinogen
- Caustic
- Oxidizing
- Teratogen
Answers are locked. Please click here to buy them.
5.58)
Which of these is a known carcinogen?
- Benzidine
- Eosin
- Hematoxylin
- Methylene blue
Answers are locked. Please click here to buy them.
5.59)
What component of Drabkin's solution is highly toxic?
- Acetone
- Cyanide
- Mercury
- Sulfur
Answers are locked. Please click here to buy them.
5.60)
What does mixing bleach with ammonia create?
- Chloramine
- Chlorine gas
- Chloroform
- Peroxyacetic acid
Answers are locked. Please click here to buy them.
5.61)
You find an old bottle of picric acid in the laboratory. The picric acid is dry. There are crystals in the bottle. How should you dispose of it?
- Call a bomb disposal service
- Dilute it with water and pour it into a hazardous waste container
- Pour it down the drain
- Send it for incineration
Answers are locked. Please click here to buy them.
5.62)
Which of these is an example of PPE?
- A fire extinguisher
- A first aid kit
- A hand-washing station
- A lab coat
Answers are locked. Please click here to buy them.
5.63)
Which type of glove has the highest resistance to permeation by gases?
- Butyl
- Neoprene
- PVC
- Rubber
Answers are locked. Please click here to buy them.
5.64)
How thick is a 5 mil glove?
- 0.005 inches
- 0.005 millimeters
- 0.5 millimeters
- 5 millimeters
Answers are locked. Please click here to buy them.
5.65)
What is the correct order of removal of PPE?
- Gloves, gown, goggles, mask
- Gown, goggles, gloves, mask
- Gown, mask, gloves, goggles
- Mask, gown, gloves, goggles
Answers are locked. Please click here to buy them.
5.66)
In which of these situations should safety goggles be worn?
- During blood collections
- During reagent and specimen preparation
- While inspecting reagent supplies
- While transporting sealed waste containers
Answers are locked. Please click here to buy them.
5.67)
A substance with the potential to produce cancer in humans or animals is called a:
- carcinogen
- caustic substance
- nosocomial substance
- pathogenic substance
Answers are locked. Please click here to buy them.
5.68)
The three basic protective measures against radiation are time, distance, and:
- lead
- radiation suit
- shielding
- speed
Answers are locked. Please click here to buy them.
5.69)
What should be your first action in the event of an acid burn?
- Cover the burn with gauze moistened in sterile saline
- Flush the burn with water
- Go to the emergency department
- Neutralize the acid with sodium bicarbonate
Answers are locked. Please click here to buy them.
5.70)
What does ABC stand for in first aid?
- Active body control
- Airway, breathing, and circulation
- Always be careful
- Attention, beware, check
Answers are locked. Please click here to buy them.
5.71)
You find a person lying on the floor. You suspect they have received an electric shock. What is the first thing you must do before treating them?
- Check if the person is breathing
- Check if the person is still in contact with the electrical current
- Check the person's pulse
- Make a note in the incident logbook
Answers are locked. Please click here to buy them.
5.72)
What is the single most important method of preventing infection?
- Proper handwashing
- Sneezing into your arm
- Thorough cleaning of equipment
- Wearing a lab coat
Answers are locked. Please click here to buy them.
5.73)
Which of these is an OPIM (other potentially infectious material)?
- Saliva
- Semen
- Urine
- Vomit
Answers are locked. Please click here to buy them.
5.74)
What does this pictogram mean?
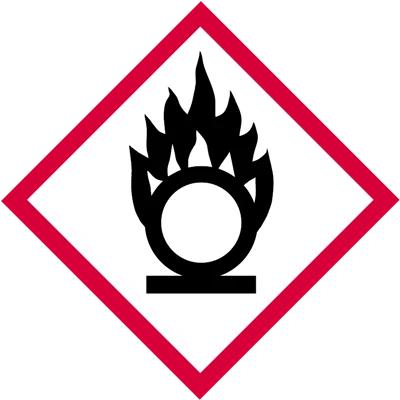
- Flammable
- Oxidizing
- Self-heating
- Self-reactive
Answers are locked. Please click here to buy them.
5.75)
Gases under pressure have a pictogram of:
- a flame
- a flame over a circle
- a gas cylinder
- an exploding test tube
Answers are locked. Please click here to buy them.
5.76)
What does this pictogram mean?

- Biohazard
- Carcinogenic
- Corrosive
- Toxic
Answers are locked. Please click here to buy them.
5.77)
Always keep picric acid:
- dry
- refrigerated
- ventilated
- wet
Answers are locked. Please click here to buy them.
5.78)
Where should bulk quantities of acetone be kept?
- Biological safety cabinet
- Flammable storage cabinet
- Freezer
- Refrigerator
Answers are locked. Please click here to buy them.
5.79)
All dangerous goods are assigned a UN number. A UN number is:
- a 4-digit United Nations number
- an 8-digit number provided by the government
- only used for dangerous chemicals
- the same as the class number
Answers are locked. Please click here to buy them.
5.80)
If the word 'Forbidden' is written in column 3 of a Hazardous Materials Table, it means the material should not be:
- discarded
- ingested
- transported
- used
Answers are locked. Please click here to buy them.
5.81)
Cold sterilization uses _________ to sterilize medical instruments.
- chemicals
- cold air
- ice
- liquid nitrogen
Answers are locked. Please click here to buy them.
5.82)
Which of these items is NOT found in biohazard spill kits?
- Autoclavable waste bags
- Disinfectant
- Petri dishes
- Safety goggles
Answers are locked. Please click here to buy them.
5.83)
Which concentration of bleach is used to decontaminate benchtops?
- 10%
- 20%
- 30%
- 50%
Answers are locked. Please click here to buy them.
5.84)
In the NFPA hazard diamond, which quadrant is for special hazards?
- Blue
- Red
- White
- Yellow
Answers are locked. Please click here to buy them.
5.85)
Which instrument is used to measure urine hydrogen ion concentration?
- Coulter counter
- Microscope
- Spectrophotometer
- pH meter
Answers are locked. Please click here to buy them.
5.86)
Which pH buffer solution is typically used first when calibrating a pH meter?
- pH 1
- pH 4
- pH 7
- pH 10
Answers are locked. Please click here to buy them.
5.87)
Which microscope is most used in clinical laboratories?
- Dark-field
- Florescent
- Light
- Phase-contrast
Answers are locked. Please click here to buy them.
5.88)
A ____________ microscope will remain in focus when you zoom in and out.
- divergent
- focused
- parfocal
- unifocal
Answers are locked. Please click here to buy them.
5.89)
What part of a microscope focuses light onto the specimen?
- Condenser
- Diaphragm
- Objective lens
- Ocular lens
Answers are locked. Please click here to buy them.
5.90)
Which objective should you begin with when focusing a microscope?
- 10x
- 40x
- 100x
- 1000x
Answers are locked. Please click here to buy them.
5.91)
Which microscope magnification is used to distinguish between different bacteria and see their internal structure?
- 10x
- 100x
- 400x
- 1000x
Answers are locked. Please click here to buy them.
5.92)
Which microscope would be best to examine an unstained, wet preparation of cells before dehydration?
- Brightfield
- Electron
- Fluorescence
- Phase contrast
Answers are locked. Please click here to buy them.
5.93)
On a microscope, what structure connects the eyepiece to the objective lens?
- Base
- Nosepiece
- Stage
- Tube
Answers are locked. Please click here to buy them.
5.94)
Microscopes usually have which objective lenses?
- 10x, 40x, and 100x
- 10x, 50x, and 100x
- 20x, 40x, and 80x
- 20x, 50x, and 80x
Answers are locked. Please click here to buy them.
5.95)
What is the total magnification of a microscope if the eyepiece lens is 10x and the objective lens is 20x?
- 30x
- 200x
- 375x
- 525x
Answers are locked. Please click here to buy them.
5.96)
Which of these glass types has the strongest resistance to heat?
- Borosilicate
- Crystal
- Optical
- Soda-lime
Answers are locked. Please click here to buy them.
5.97)
The cabinet with the highest personal, environmental, and specimen protection is the:
- class A biological safety cabinet
- class I biological safety cabinet
- class II biological safety cabinet
- class III biological safety cabinet
Answers are locked. Please click here to buy them.
5.98)
Class 1 hazardous materials are:
- explosive
- infectious
- poisonous
- radioactive
Answers are locked. Please click here to buy them.
5.99)
Which act gives workers the right to know about hazards in the workplace?
- Health Care Consent Act
- Occupational Safety and Health Act
- Privacy Act
- SAFETY Act
Answers are locked. Please click here to buy them.
5.100)
Which type of microscope is best for viewing crystals in a sample of joint fluid?
- Fluorescent light
- Light
- Polarized light
- Ultraviolet
Answers are locked. Please click here to buy them.
Access options
Get online access or buy a physical book.
Option 1: Online access
Unlock the answers to 250 questions for just $39.99
You'll get immediate access after payment.
Step 2
Select one of the payment buttons below.
Option 2: Physical book
Get the questions as a book
The questions and answers are available as a book at Amazon.com

 Examelot
Examelot

